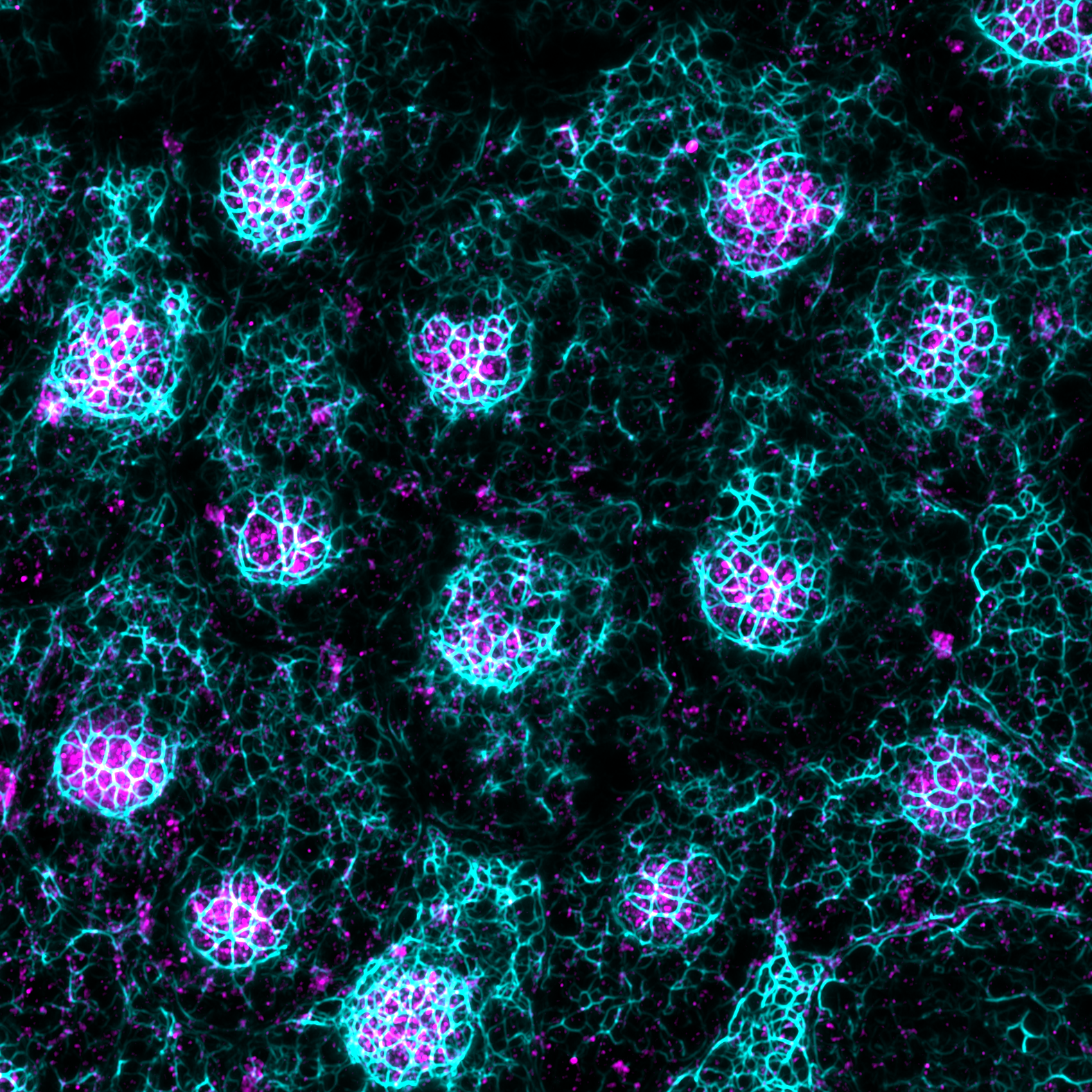
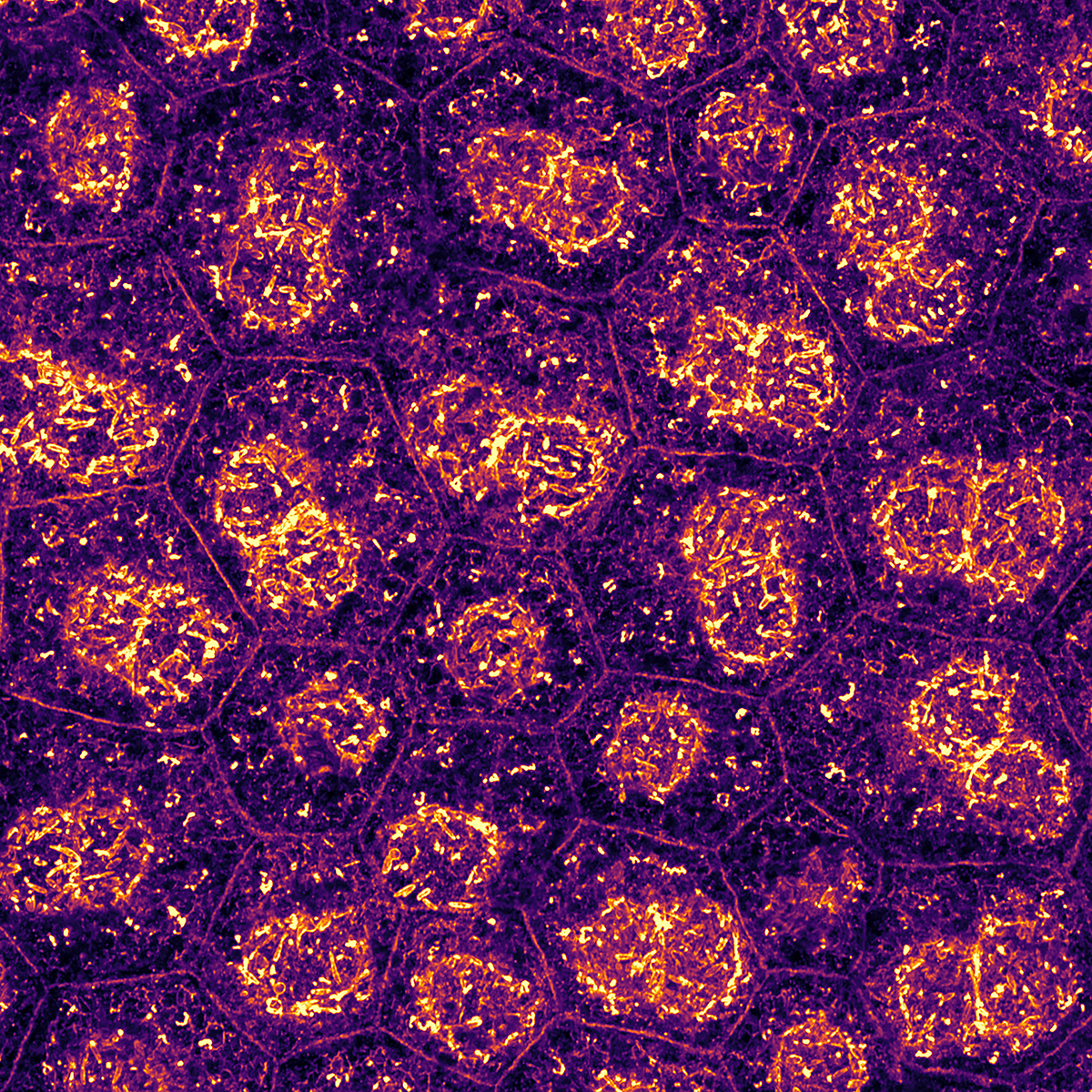
Di Russo's research group
REMeD
How Do Mechanics Shape Tissue Function?
Cells continuously sense and respond to mechanical cues that shape their structure, organization, and communication. These forces regulate tissue architecture, biochemical signaling, and key processes like growth, repair, and homeostasis.
Mechanobiology explores how cells interpret and generate mechanical forces, converting them into biochemical signals that drive tissue function and adaptation.
By studying tissue mechanobiology, we aim to uncover medically relevant insights and engineer advanced in vitro models that bridge fundamental research and clinical applications.
An Interdisciplinary Approach
Our research focuses on uncovering the role of cell-extracellular matrix and cell-cell communication in mechanobiological processes.
By combining cell biology, chemistry, and physics, we investigate how mechanical forces regulate retinal physiology and pathology, an area often overlooked in traditional studies.
Moreover, addressing the lack of human-based models, we are developing advanced systems that merge stem cell technologies with precisely engineered hydrogels.
Our Current Projects